
Click on the image of the Periodic Table of the Elements below to get a larger image that has the name, as well as the symbol, of each element. This is the way the table is usually drawn, with the "lanthanide" and "actinide" subsets (elements 57-70 and 89-102 respectively) shown on separate rows rather than in the main table; as can be seen below, the table gets awfully wide if this is not done!
The periodic table of the elements was originally devised in order to organize the various elements into groups having similar chemical properties, represented by the vertical columns of the table. Chemistry is what happens when atoms share or swap electrons, so these properties are in turn determined by the arrangement of the electrons that surround the nucleus of the atom. 2019 is the International Year of the Periodic Table, and in observance of the occasion I put together this webpage to show how the structure of the electrons gives rise to the structure of the table. Actually, I got the idea for this page when discussing the topic with my daughter in the context of her 10th grade chemistry class; I could not find a sequence of diagrams like that below online, so I hope that this will be of use to other chemistry students and their teachers.

The pair of diagrams above shows how to fit together the electrons for all the elements discovered or synthesized to date. They are pretty complex, like the Periodic Table itself, but they are the result of a few simple patterns that make more sense when we build the table up period by period, as the rows are called. Actually, those "simple patterns" result from quantum physics, which is a lot heavier going than 10th grade chemistry; it is typically an upper-division college course for physics majors, and I'm going to have to just spell out those patterns as assumptions rather than deriving them (not least because it has been decades since I took that course, and couldn't do the derivation from memory if I tried!). I'll give a few paragraphs of explanation here to describe what you're looking at, but again it makes more sense when built up row by row as we'll do below.
The stacks of lines in the diagram at left represent the energy levels of the electrons of an atom. Unlike, say, the planets in the solar system, the electrons orbiting the nucleus of an atom are not found at arbitrary distances from the center and at arbitrary orbital speeds around it, and thus at arbitrary energies; rather, they are constrained to be in certain "quantum states" with specific energies. That is, there's no reason there couldn't be a planet halfway between Earth and Mars, with intermediate energy of motion and gravitational potential energy; there just doesn't happen to be such a planet in our solar system. This is not the case for electrons: an electron cannot orbit a nucleus in a way that places it between two of the atom's energy levels. (This means, in turn, that an electron can't lose an arbitrary amount of energy and move to another, slightly different orbit; rather, it makes a "quantum jump" from one energy level to another and releases a specific amount of energy, usually as an escaping photon or light particle. The existence of this specific "quantum" of escaping energy, rather than just arbitrary amounts each time this process occurs, is what gave quantum theory its name in the first place.)
Each differently colored stack of lines represents a "shell" that corresponds, roughly, to how far an electron in that shell is from the nucleus on average. As with gravitational orbits in a solar system, an electron has lower energy if it orbits closer to the center, so the lower-energy shells at the left in the diagram are closer to the nucleus. Within each shell are a fixed number of "orbitals" that correspond, again roughly, to how fast the electron is going around the nucleus. As you can see, there is one orbital in the lowest shell, two in the next, three in the next, and so on; this is one of the simple patterns I referred to above. The orbitals within a shell have different energies, as shown qualitatively in the diagram, and the relative positions of the energy levels of orbitals in different shells are also shown, as will be spelled out below. The dashed lines at the upper right of the diagram are orbitals that are not populated in any of the elements discovered or synthesized so far, so I don't know how to put their energies in order. (Presumably this could be calculated, but that would require some really advanced quantum physics!)
The diagram at right above shows how individual electrons fit into the energy levels diagrammed at left. Again, there is a simple pattern: there can be up to two electrons in the first orbital of each shell, six in the second, ten in the third, fourteen in the fourth, and so on, adding four for each higher orbital. (I don't show any orbitals past the second in the seventh or outermost shell, drawn in magenta, because they are unoccupied for any of the atoms in the table at top.) The orbitals, from lowest to highest, are commonly labeled s, p, d, f, and after that g, h, etc., and I'll refer to them as such. According to this historical article, these labels came from early spectroscopic examinations of atoms, and stood for sharp, principal, diffuse, and fundamental, with g, h, etc. added later as more orbitals were needed to explain atoms with more electrons.
An atom consists of a nucleus made up of positively charged protons and uncharged neutrons, about which the negatively charged electrons orbit. In an electrically neutral atom there must be an equal number, usually labeled Z, of electrons and protons; this is the number of each element in the table at top. (The number of neutrons can vary for a given Z, but they don't affect the discussion below since they have no electrical charge, and so I'll ignore them.) The electrons in an atom will fill up orbitals and shells from the lowest energy to higher energies, filling out the spaces in the diagram at right in the order spelled out by the level diagram at left. The discussion below shows how this all fits together, period by period (row by row).


The first period is simple: the lowest shell has only the s orbital, which holds two electrons, so there are two elements: hydrogen and helium. The colored (here, black) bars in the element symbols are the same as those used for the shell being filled as we progress along the row. This pattern continues below.
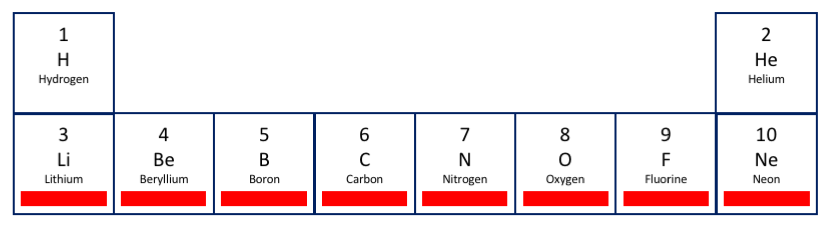

As I mentioned, the periodic table was originally devised to sort elements into groups (columns) with similar chemical properties. The rightmost column is the "noble gases," so called because they are the least reactive of the elements -- think of a snooty nobleman who doesn't associate with more common folk. (They don't even mingle with each other much, so these elements remain gaseous instead of liquefying down to extremely low temperatures.) The discussion above of the energy levels of orbitals was a bit oversimplified: the exact energy of an electron varies not only by shell and orbital but also by how many of the available spaces in that orbital are occupied, and orbitals that are completely filled have lower energy, i.e., are more stable, than other configurations. This explains the inertness of helium, which has the single s orbital of the lowest shell filled; in the second and subsequent periods, though, the most tightly bound configuration has the s and p orbitals filled in the outermost shell, so these elements make up the column of the noble gases below helium. For the second period, this adds eight more electrons (and protons) for elements 3-10.
If the s shell alone is filled as for beryllium (Z = 4) in the second period, or has one electron as for lithium (Z = 3), these two or one electrons are comparatively loosely held and the element ionizes (gains or, in this case, loses electrons) easily to strip down to the electron configuration of the next lower noble gas. That is, past the first period the filled s orbital is not especially inert. This baseline on which each period is built is indicated in this and subsequent energy level diagrams by the dotted line showing the orbitals filled in the earlier periods, and a label identifying the noble gas with that electron configuration from the previous period. In the diagram of circles at the right, the previously filled orbitals are denoted with dark gray electrons.


The third period also adds eight elements to reach the next noble gas, filling the s and p orbitals of the third shell (yellow). Note that this shell has the d orbital too; this is not filled in this period, so it is drawn with dashed lines in the two diagrams above.
As the rows get longer the table gets wider, so here and subsequently you can click on the image of the table to get a larger image that has the names of the elements.

If orbitals were ordered in energy in a straightforward way, so that all those of one shell filled before those of the next began to be filled, the periodic table would look more like a staircase. In reality, the energy levels are arranged so that the s orbital of the fourth shell (denoted 4s) is at lower energy than the final orbital of the third shell (3d), and so elements 19 and 20 place two electrons in the 4s shell, then the 3d shell fills, and finally the 4p shell fills to end the period with a noble gas (again leaving the 4d and 4f shells empty and shown by dashed lines). The table is split as shown so that the elements with the same electron configuration in the outer shell (with s or p orbitals being filled) are below one another in each period; this "valence" shell is where the action of chemistry takes place and, again, the configuration of electrons determines the element's chemical behavior, which is how the periodic table was put together in the first place.

The fifth period repeats the pattern of the fourth: 5s fills, then 4d, then 5p. Thus elements 37-54 fall into place below elements 19-36 one period up. This leaves one orbital, 4f, empty in the fourth shell; three remain to be filled in the fifth shell (5d, 5f, 5g).

With all the empty orbitals below the valence shell in the sixth period, another split is introduced in the table as shown. It's really getting wide now, which is why these new elements are usually cut out and drawn separately, as at the top of this webpage. First 6s fills, then 4f is finally filled, then 5d and at last 6p.

As with 2 to 3 and 4 to 5, the pattern of period 6 repeats in period 7: 7s, 5f, 6d, 7p. The last element with any stable isotopes (combinations of protons and neutrons in the nucleus that don't spontaneously decay over time) is bismuth (Z = 83), and the last naturally occurring element is uranium (Z = 92); all elements with higher Z have to be synthesized in a nuclear reactor or particle accelerator. Since most of these elements decay very quickly, samples large enough to enable tests of their chemical properties are impossible to collect, and their places in the periodic table have to be determined entirely by their calculated electron configurations rather than by the original technique of comparing chemical properties.
Well, not quite. As I mentioned, the exact energy of an electron depends on the number of electrons in a partially filled orbital, rather than being exactly the same for all the electrons in that orbital. For example, given the discussion above one would expect copper (Cu, Z = 29) to have a filled 4s orbital and nine electrons in the 3d orbital. However, since orbitals really "want" to be filled (lower energy, more stability), instead the 3d orbital is filled with ten electrons and the 4s shell is back to one, losing the tug of war in this case. Several other elements have similar rearrangements.
The "official" names of elements are determined by the International Union of Pure and Applied Chemistry (IUPAC). That is to say, its recommendations do not have the force of law anywhere, but professionals in the field of chemistry use its terminology so that everybody can understand each other. After an element is discovered or synthesized, a provisional symbol and name will be assigned until a formal decision is made on the final ones. For example, until the name Nihonium and symbol Nh were finalized for element 113, it was given the temporary symbol Uut and name Ununtrium, which might literally be translated as "one one three-ium"!
Good question! With element 118, oganesson, the seventh period of the periodic table is complete, so an eighth row will need to be started. It's probably a safe bet that elements 119 and 120 will fit in the first two columns below elements 87 and 88; however, after that it depends on the ordering in energy of the dashed lines in the energy level diagram, relative to each other and to the levels in the eighth shell. If I had to guess, based on the pattern that one shell deeper starts to fill with every other period, I'd think that element 121 would start filling the 5g shell, then 6f, 7d, and 8p would follow. If that's the case, the table would again have to be split between elements 56 and 57 and between 88 and 89, and eighteen new columns for the electrons of the g shell would have to be inserted before element 139 falls below element 89! As I noted, though, calculating this would be a matter of advanced quantum physics, well beyond anything I could answer definitively.
 Back
To Home Page
Back
To Home PageAll content copyright 2019-2024 by Mark Looper, except as noted. Reuse of my copyrighted material is authorized under Creative Commons Attribution 4.0 International license (CC BY 4.0).
![]()
![]()
New 3 January 2019